Research
I. Theory and Modeling of Photovoltaic Materials
Chile has a tremendous energy dependence on foreign resources, with a demand on oil and natural gas that reach around 80%. This energy dependence has started a strong debate about the need to expand our energy matrix. The negative impact of fossil fuel and nuclear power on the environment have focused the discussion on increasing the hydroelectric power and developing alternative energy sources like wind and geothermal. On the other hand, our country receives the highest solar radiation in the world by the Atacama Desert, with an irradiance of 275 W/m2. Despite this favorable scenario, very little is being done to create new knowledge to exploit this free and unlimited energy resource. Noncovalent functionalization of single-walled carbon nanotubes (CNTs) with photoactive molecules is becoming a promising technique to explore functional materials for light-harvesting or optoelectronic applications. The coupling of the exceptional CNT transport properties with the optical properties of functional dyes, like porphyrins with a strong absorption at the near-UV and visible regions, makes CNT/dye complexes good candidates for sensitive nanoscale devices with potential applications in hybrid organic–inorganic photovoltaic devices.
II. Theory and Modeling of Novel Materials for Catalysis and Energy Conversion
The increasing demands for energy sources that avoid the emission of carbon dioxide and other pollutant to the environment have focused the attention worldwide in the possibility to use hydrogen as a long-term solution for clean and renewable energy sources. However, there exist an enormous gap between a future hydrogen economy and our current fossil-fuel economy. The only hope of narrowing this gap significantly is to find innovative solutions to the fundamental problems of hydrogen production and storage, which can only be achieved from basic research. Conversion of hydrocarbon fuels (oil or natural gas) to hydrogen with a high degree of purity for proton-exchange-membrane (PEM) fuel cell operation represents a key challenge for the design of low-cost catalysts for the oxygen reduction reaction (the breaking of the O2 molecule), which is the rate limiting process. In addition, catalysts with higher activity, specificity and stability are needed for reducing kinetic barriers and increasing the thermodynamic efficiency of many different reactions in the hydrogen production. Recent developments in nanotechnology have opened new opportunities for the controlled synthesis of nanomaterials with tailored structures. In this respect, electronic structure calculations are becoming a fundamental tool of investigation in nanostructured materials. These methods are based on the solution of the non-relativistic Schrodinger equation without any empirical input, the so called ab initio methods, and have the power to describe entire reactions in extended systems with “chemical” accuracy.
Recent projects:
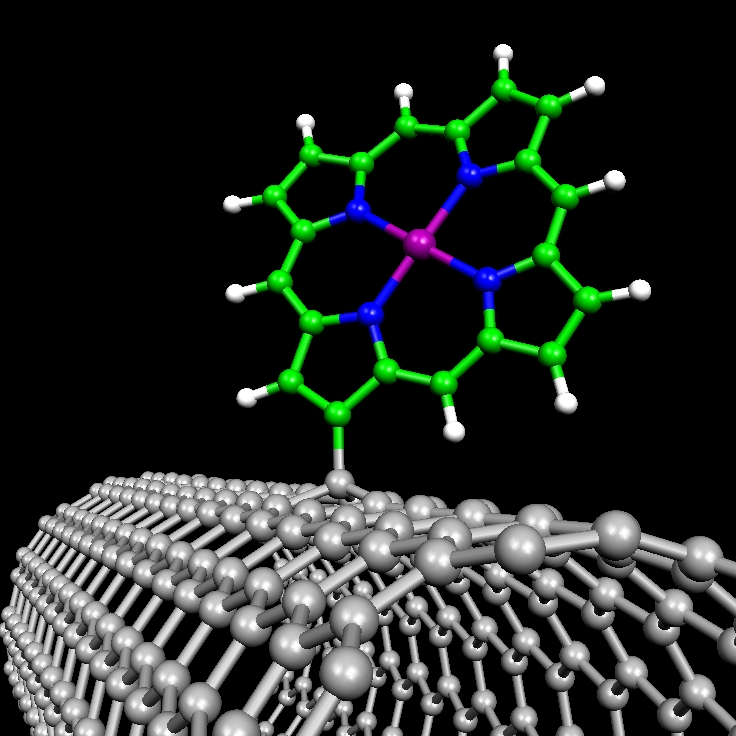
Transition metal porphyrins (MP) and phthalocyanines (MPc) attached on carbon nanotube sidewalls. We explore the covalent attachment of MP and MPc macrocycles, with M = Mn, Fe, and Co, on the surface of metallic single-walled carbon nanotubes (CNTs). We study the stability and electronic properties of these assemblies to shed light in the experimentally reported electrocatalytic activity of carbon-supported Fe and Co macrocycles and the role played by the linking mechanism. The catalytic activity for the oxygen reduction reaction of these supramolecular complexes is currently under investigation.
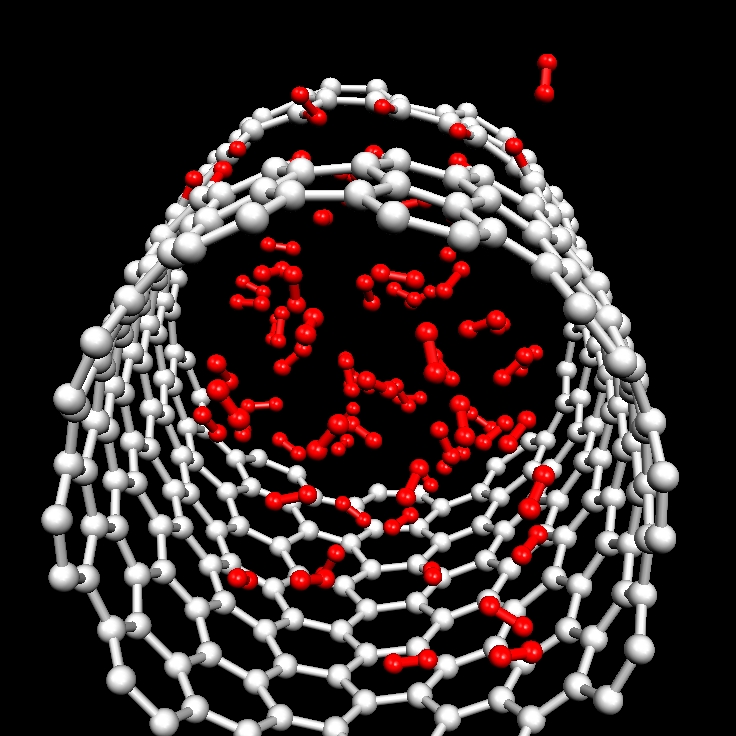
H2 storage inside single-walled carbon nanotubes. The interaction of hydrogen molecules with multivacancy defects in CNTs and their subsequent incorporation inside at room temperature are investigated by ab initio molecular dynamic simulations. We find endohedral binding energies for H2 close to those estimated optimal for a reversible adsorption-desorption process, suggesting that nanoporous CNTs as produced by electron irradiation could be and effective hydrogen storage medium, allowing the access to the CNT inner space. Storage capacity of about 4 wt% are estimated for a 11 Ang. CNT, with the possibility to be increased for CNTs with larger diameters [Phys. Rev. B 80, 075421 (2009)].
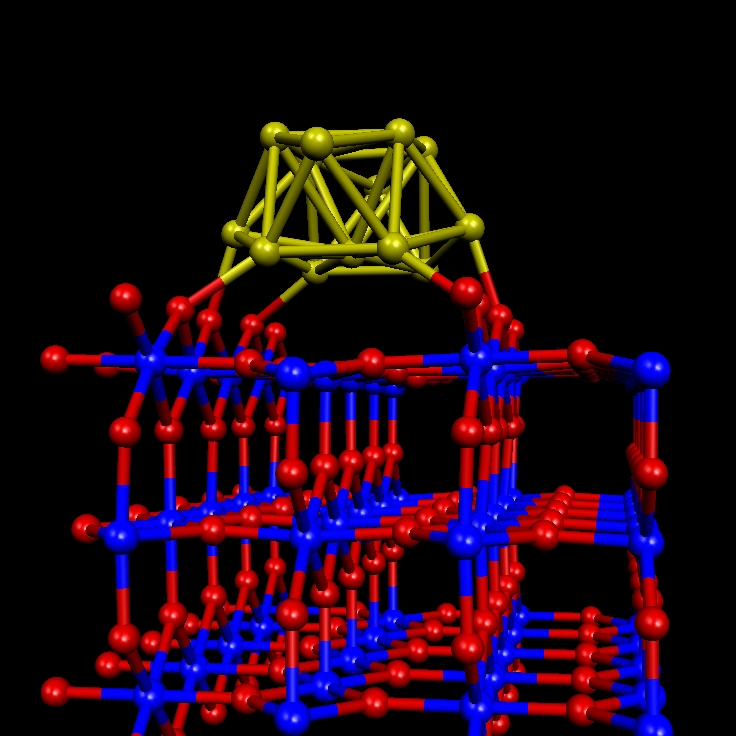
Metallic nanoparticles supported on metal oxide sufaces. The electronic properties of metallic nanoparticles (Au, Pd, Pt) supported on metal oxide surfaces like TiO2(110) and CeO2(111) are key to understand the superior catalityc activity that some of these systems exhibit for the CO oxidation and O2 reduction reactions. Different parameters which may be involved in the catalytic activity are investigated, including the metallic species of the particle, its size and the role played by surface defects, particularly oxygen vacancies, as a possible site for the nanoparticle adsorption and (de)activation.